【Article Introduction】
Professor Jun Wan from the School of Chemistry and Chemical Engineering at Wuhan Textile University recently published a paper titled “Harnessing the Unconventional Cubic Phase in 2D LaNiO3 Perovskite for Highly Efficient Urea Oxidation” in Angewandte Chemie International Edition. Wuhan Textile University is the primary affiliation of this groundbreaking work.
The study introduces a novel microwave thermal shock method to induce phase transitions in 2D LaNiO3, enabling the transformation between the conventional hexagonal phase and unconventional cubic and triangular phases. The rapid quenching process preserved the material's 2D porous structure. Among these, the cubic phase exhibited superior symmetry and larger interlayer spacing, which significantly enhanced electron transport and accessibility of active sites, resulting in outstanding urea oxidation reaction (UOR) performance. This is the first demonstration of LaNiO3 leveraging unconventional phase regulation to optimize the hybridization between Ni 3d and O 2p orbitals and the local charge distribution, effectively boosting the catalytic performance of the six-electron transfer process. This work highlights the unique advantages of microwave thermal shock in controlling phase transitions in 2D materials, while offering new insights into the influence of phase engineering on electrocatalytic mechanisms.
【Research Background】
Phase engineering is a critical strategy in electrocatalysis for optimizing the electronic, geometric, and chemical properties of materials. However, achieving phase transitions in thermodynamically stable perovskite materials, especially within the constraints of 2D structures, remains challenging. LaNiO3 is a classic perovskite material whose electrocatalytic performance is closely tied to its crystalline phase. While the hexagonal phase of LaNiO3 demonstrates moderate catalytic activity, its conductivity and interlayer distance limit the diffusion and reaction efficiency of urea molecules. Introducing unconventional phases, such as the cubic phase, can enhance electron transport and accessibility of active sites, thus improving catalytic performance. Traditional high-temperature synthesis methods, however, struggle to precisely control phase transitions in 2D structures, adding complexity to phase engineering. The development of a rapid high-temperature reaction strategy is therefore critical for designing 2D LaNiO3 and high-performance UOR catalysts.
【Key Findings】
Thermodynamic analyses revealed stability differences among hexagonal, cubic, and triangular phases, with the cubic phase demonstrating the highest stability and optimal electronic transport properties. Density functional theory (DFT) calculations further showed that phase transitions significantly modulate interlayer spacing, thereby enhancing charge transfer efficiency and reaction performance.
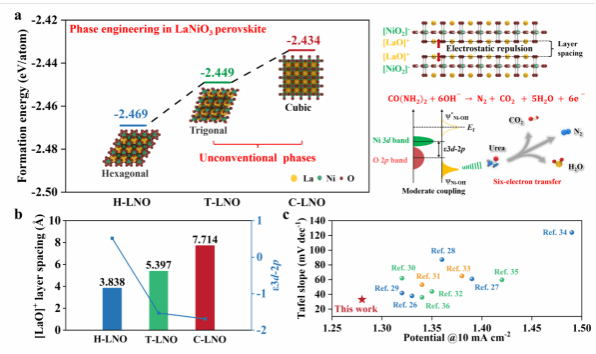
Figure 1. Structural Features and Optimization Mechanisms.
Compared to traditional high-temperature annealing methods that lead to particle aggregation, the microwave thermal shock method successfully synthesized 2D porous LaNiO3 structures in hexagonal, triangular, and cubic phases. SEM and HRTEM images confirmed the uniformity of these structures.
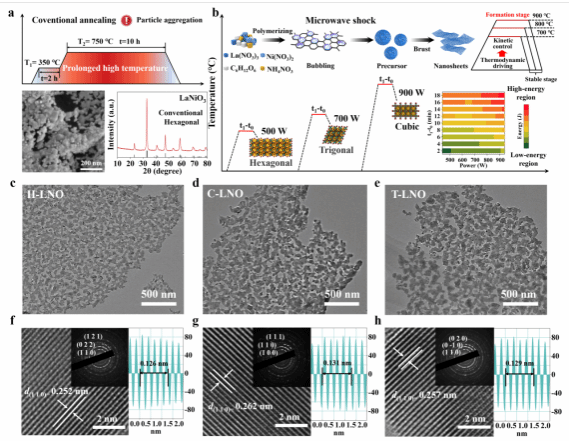
Figure 2. Morphological Characterization.
XRD and Rietveld refinements revealed significant variations in Ni–O bond lengths and angles among the phases. Jahn-Teller distortion in the NiO6 octahedra and changes in local electronic states were identified as key factors influencing structural stability and catalytic performance.
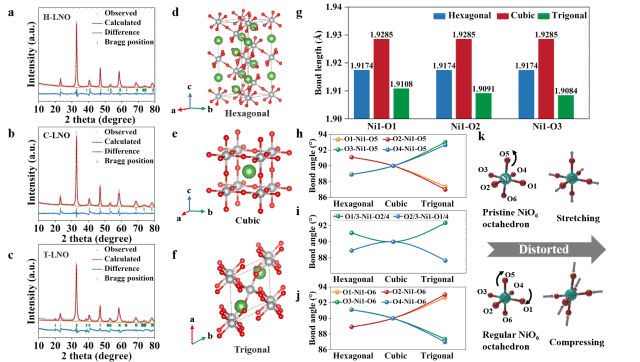
Figure 3. Phase Transition and Structural Reconstruction.
The cubic-phase LaNiO3 (C-LNO) exhibited superior catalytic activity in 1 M KOH and 0.33 M urea, with the lowest onset potential (1.28 V) and remarkable current density improvements. Tafel slope analysis demonstrated C-LNO's fastest reaction kinetics (33.1 mV dec-1). Electrochemical impedance spectroscopy revealed reduced reaction resistance and enhanced charge transfer capabilities in the cubic phase.
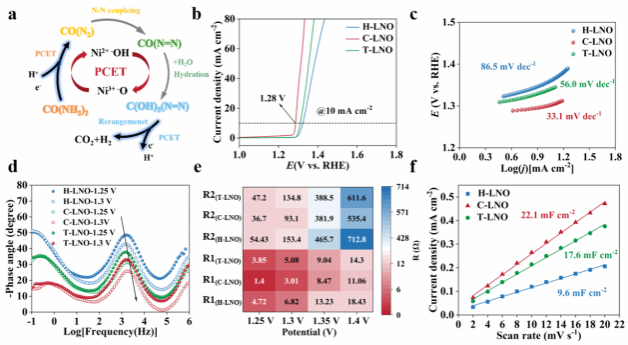
Figure 4. Urea Oxidation Performance.
XPS analysis indicated the highest concentration of surface oxygen vacancies in the cubic phase, followed by the triangular and hexagonal phases. EPR spectra corroborated this trend. UV-Vis spectroscopy showed a significantly narrower bandgap for the cubic phase (3.21 eV) compared to the hexagonal (3.83 eV) and triangular (3.52 eV) phases, supporting enhanced conductivity. DFT calculations further elucidated how oxygen vacancies and the hybridization between Ni 3d and O 2p orbitals influenced catalytic performance.
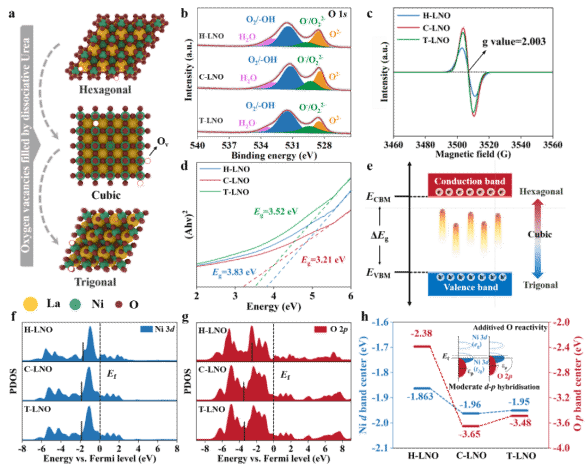
Figure 5. Oxygen Vacancies and Electronic Structure.
This study provides an efficient synthesis strategy for inducing phase transitions in 2D materials, substantially enhancing their catalytic performance. The findings lay a theoretical foundation for phase engineering and offer new directions for exploring structure-regulated mechanisms. Further optimization of the microwave thermal shock method and exploration of phase transitions in other 2D materials could unlock even greater potential in catalysis and related fields.
【Article information】
Harnessing the Unconventional Cubic Phase in 2D LaNiO3 Perovskite for Highly Efficient Urea Oxidation
Zhiao Wu,#,a Miao Fan,#,a Huiyu Jiang,#,a Jiao Dai,a Kaisi Liu,a Rong Hu,b Shutong Qin,a Weilin Xu,a Yonggang Yao,*,b and Jun Wan*,a
Angewandte Chemie International Edition
DOI:org/10.1002/anie.202413932


